In-House Cellular Biotechnology Laboratory
Each mesenchymal stem cell (MSC) preparation is released only after a ≥ 98% live-cell count is confirmed at release with validated viability assays under controlled conditions.
Why Viability at Release Matters
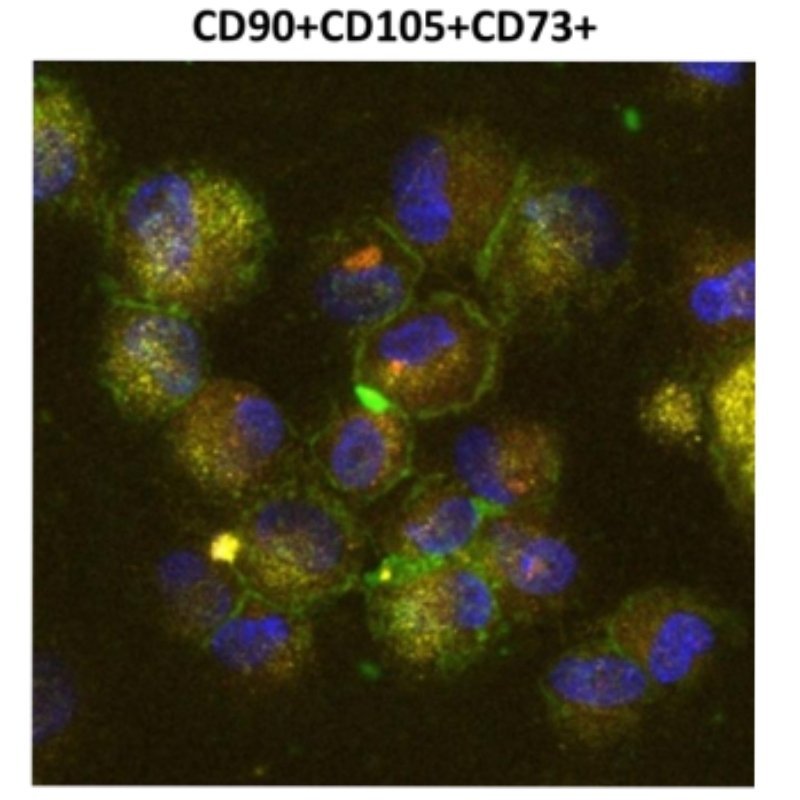
In MSC culture and preparation, quality is determined at the moment of release, not upstream. Viability is the proportion of living, metabolically active MSCs available to perform their biological functions. Because handling, time and temperature can affect it, our in-house laboratory verifies MSC viability at release using validated assays—including flow-cytometry viability gating when appropriate—and clears a preparation only when a defined release threshold is met. Viability is a process quality attribute that supports consistency and dose accuracy. Other pillars—identity/purity (e.g., CD73/CD90/CD105 profile) and sterility/safety—are evaluated separately under our quality system
- Measured at release with validated assay
- Non-release policy for out-of-spec batches
- Dose check aligned to actual live-cell content
Quality & Release Standards (QRC)
Quality is verified at release across four controls. These are process quality attributes that govern clearance for physician-led administration.
Identity & Purity
We confirm the intended stromal precursors (MSC) and exclude undesired lineages under internal acceptance ranges. Results are recorded in the clinical file.
Sterility, Endotoxin & Mycoplasma
Each batch completes sterility testing, endotoxin review against limits, and mycoplasma screening before release. Out-of-spec batches are not released.
Dose Verification
Before release, we verify alignment between the intended dose and the quantified live-cell content.
Documentation & Patient Access
Each preparation carries a unique lot code linked to processing and QC records retained under SOPs. At the patient’s request, we issue a Viability Certificate stating the live-cell value measured at release.
Cell Sources & Preparation Framework
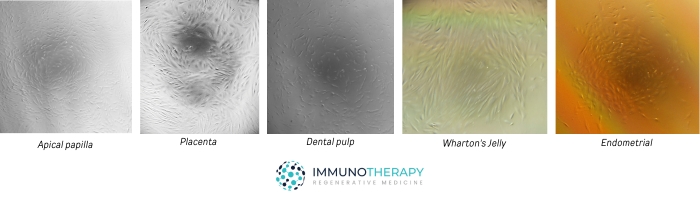
We culture and prepare mesenchymal stem cells (MSC) in-house from validated tissue sources—apical papilla, placenta, dental pulp, Wharton’s jelly, endometrial tissue, adipose tissue, and periodontal ligament. Each preparation is released only after identity/purity and safety checks are completed.

Regulatory Authorization & Patient Safety
Our facility operates under a COFEPRIS license and maintains documented quality-assurance systems. All MSC preparations undergo in-house testing for viability, identity and sterility and are released only after meeting predefined quality criteria.
Advantages of Our Laboratory

No Cell Freezing
We avoid cryopreservation and work with non-cryogenic, freshly released mesenchymal stem cell (MSC) preparations. By omitting thawing and associated washes, we reduce handling, minimize pre-procedure variability, and preserve cell integrity through use. Release is performed with pre-use quality controls and end-to-end documented traceability.

Multidisciplinary Team
Physicians and laboratory specialists operate as a single clinical-technical unit. This results in a single chain of custody, consolidated documentation, and deliveries synchronized with the procedure, reducing rescheduling and avoiding last-minute surprises. The focus is practical: have the correct, labeled, and verified preparation at the right moment.

Personalized Cell Preparation
Each case is configured for mesenchymal stem cell (MSC) preparations according to the medical protocol—specifications for dose, volume, and release timing. Labels include the patient ID and unique lot code; records are aligned to the clinical chart. If scheduling adjustments arise, our in-house operation enables re-synchronization without compromising release specifications or traceability.